How to Apply
Most grant categories have an application form with specific instructions on how to complete the application. If your grant does not have an application form, please follow these general submission guidelines.
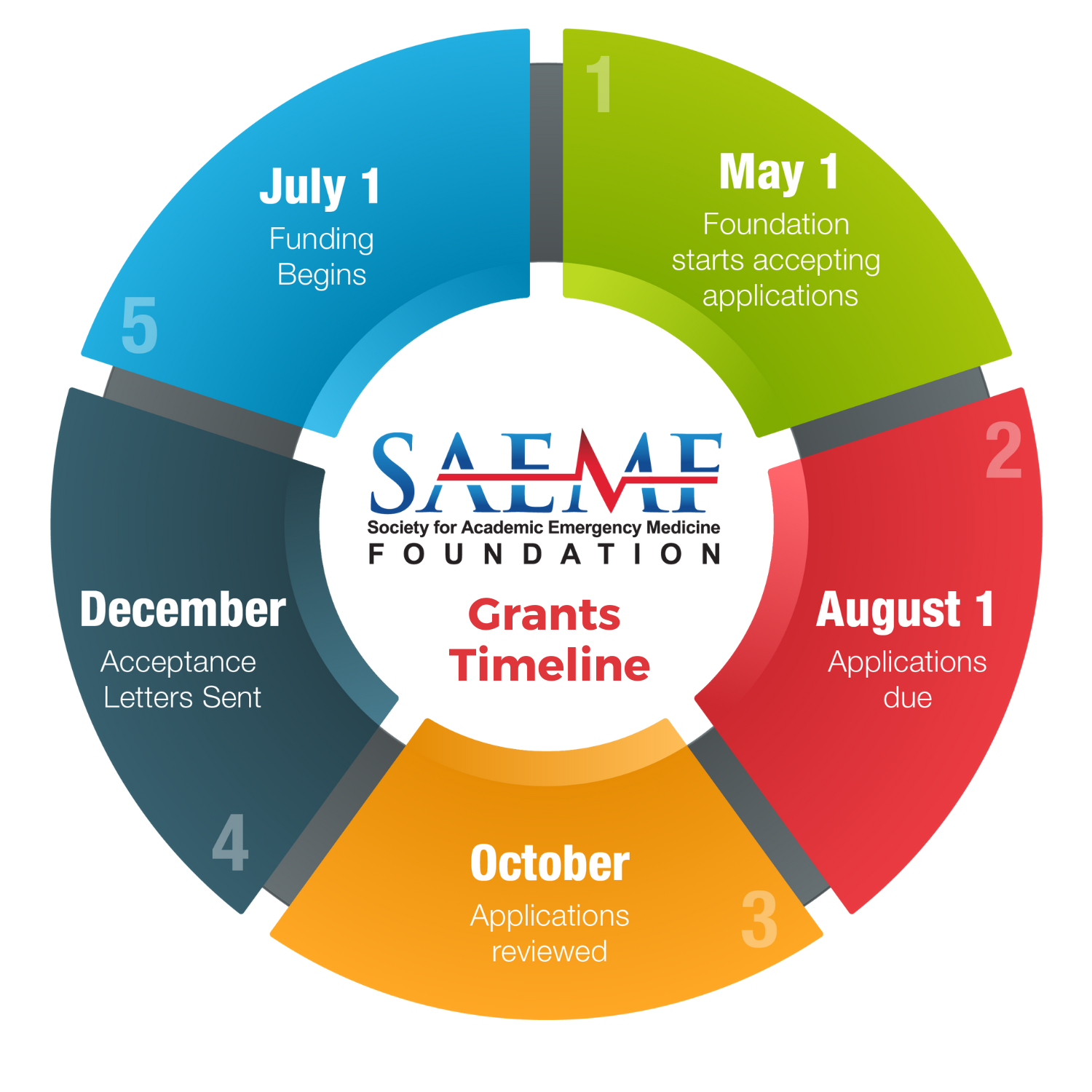
SUBMISSION GUIDELINES
- All applications must be submitted electronically through the SAEM Foundation Grant Portal, including letters of recommendation or support. All applications must be received on the due date by 5pm Central.For information on how to use the SAEM Foundation Grant Portal please view the Applicant Instructions.
- Please visit our Grants FAQ page for questions regarding the application process.
- If you need further assistance after viewing the FAQs, you may send your specific questions to grants@saem.org.
OTHER POLICIES AND PROCEDURES
Grant Review Process
The Grants Committee is responsible for the review of all applications, and will make recommendations to the SAEM Board of Directors regarding selection of recipients. A scoring system considering the qualifications outlined in the grant application will be assessed with the goal of funding applications with the highest likelihood of training, or becoming, highly productive academic emergency physicians in the specialized area of expertise. Grants will be awarded only if a project deemed suitable and worthy of the award is submitted during that grant cycle.
Policy on SAEM Grant Awards to Previous Grant Recipients
The SAEM Grants Committee is dedicated to ensuring a process which best utilizes grant funds and promotes research which will be useful to academic emergency medicine and public health at large.
In reviewing grant applications, the Grants
Committee will determine whether each applicant, including any named researcher involved in the application, has received SAEM grants in the past.
If grants have been received, the Grants Committee shall review the available files to determine
whether the applicant complied with the Grant Agreement, including but not limited to whether the quality of the work done was that expected by SAEM, whether the required reports were submitted in a complete and useful form by their respective deadlines,
whether the books and records maintained were in the form required by the Grant Agreement, and whether all other terms and conditions of the Grant Agreement were followed.
The experience of the Grants Committee and SAEM with the applicant
shall be a primary consideration as to whether the new application should be accepted. If the applicant has failed to follow the prior Grant Agreement in any material respect, the applicant shall be ineligible to receive a subsequent grant.
Policy on Overlapping Funds
The SAEM Grants are designed to support applicants in their research careers. A SAEM grantee with any overlapping or potentially overlapping support (support for the same purposes from two different funding agencies) should report such overlapping
support to the SAEM Grants Committee Chair once this occurs. SAEM reserves the right to adjust the grant award pending any overlapping support.
A subcommittee of the SAEM Grants Committee will review any cases of overlapping support
or potential overlapping support. This subcommittee will consist of the Grants Chair, the Grants BOD representative, the subcommittee chair for the particular grant, and two members at large from the Grants Committee.
Policies Governing the Use of Humans and Animals
If human or animal experimentation is to be performed during the period of the award, the department and institution to be used as the training site must affirm that: (1) investigations involving human subjects proposed and subsequently carried out in the application have been endorsed by the Institutional Review Board, or other clearly designated appropriate body, at the study site; (2) any research involving human subjects will conform ethically with the guidelines prescribed by the National Institutes of Health (NIH); and (3) research involving animals will conform with the current “Guide for the Care and Use of Laboratory Animals”, NIH publication, DHHS/USPHS, and has been approved by the Animal Care and Use Committee at the study site. Distribution of funds will be dependent on Human Subjects or Animal Welfare assurance approval for the proposed research study.
Investigatory Financial Disclosure Policy/Objectivity in Research
Principal investigators and institutions are required by SAEMF to comply with the PHS regulations, Final Rule, 42 CFR Part 50, Subpart F, Responsibility of Applicants for Promoting Objectivity in Research. The signature of the officials signing for the principal investigator's and mentor's institution on the Signature page of the application indicates compliance that an institutional administrative process is in effect to identify and resolve conflicting financial interests of the type described in Subpart 50.604(a) with respect to all projects for which funding is sought and awarded from SAEMF.
Patent, Intellectual Property, and Technology Transfer Policy
All inventions discovered or arising out of research supported in whole or in part by SAEMF, which may be used in emergency medicine research, teaching, or practice, shall be reported within one (1) year to SAEMF. "Invention" is any discovery, material, method, process, product, program, software of use, whether or not patented or patentable or copyrighted or copyrightable, that has application of value such that its use, licensing, lease, or sale can generate revenue.
If the institution receiving or disbursing SAEMF funds, which supported the invention has an established and applicable patent, intellectual property, or technology transfer policy and procedure for administering inventions, SAEMF will defer to that policy.
If the institution has no established and applicable patent, intellectual property, or technology transfer policy and procedure for administering inventions, SAEMF shall have the right to determine the disposition of invention rights. In such cases, SAEMF may:
- Decide that patent and copyright should be or not be filed;
- Release the invention to the inventor(s) or inventor's designee;
- Submit the invention to a qualified organization for administration and licensing;
- Determine by negotiation the fair share of royalty income to be paid to the inventor(s);
- License or make other arrangements for the application and use of the invention on an exclusive or non-exclusive, royalty, or royalty-free basis.
Investigatory Financial Disclosure Policy/Objectivity in Research
Principal investigators and institutions are required by SAEM to comply with the PHS regulations, Final Rule, 42 CFR Part 50, Subpart F, Responsibility of Applicants for Promoting Objectivity in Research. The signature of the officials signing for the principal investigator's and mentor's institution on the Signature page of the application indicates compliance that an institutional administrative process is in effect to identify and resolve conflicting financial interests of the type described in Subpart 50.604(a) with respect to all projects for which funding is sought and awarded from SAEM.
Policy on Transfer of Awards
SAEMF grants are competitive grants awarded based on merit of the applicant, mentor, and institution. This policy addresses general circumstances that may arise in the event of change of institution by either the applicant or mentor or both.
If the applicant or named mentor changes institutions or ceases research in the field for which the award was made, in most cases the award will terminate and the remaining balance will be returned to SAEMF. If exceptional circumstances are present, the award recipient can submit a formal, written document outlining the circumstances and why funding should not be withdrawn. However, in the case of the mentored grants, the new mentor must have been a co-applicant on the original submission and have experience in the same field of study. This request should be a formal request in writing and should contain a compelling reason for continuation of funding.
If the named applicant changes institutions or ceases research in the field for which the award is made, in most cases the award will terminate and the remaining balance will be returned to SAEMF. If exceptional circumstances are present, the award recipient can submit a formal, written document outlining the circumstances and why funding should not be withdrawn. This request should be a formal request in writing and should contain a compelling reason for continuation of funding.
These situations will be considered on a case-by-case basis by the SAEMF Grants Committee.
