Rhythm Interpretation
Objectives:
- Understand the basic concepts of pediatric (electrocardiogram) ECG interpretation
- Differentiate pediatric ECG interpretation from ECG interpretation in adults
- Describe the pathophysiology that lead to the pediatric ECG findings
- Describe arrhythmias and cardiac anomalies seen in the pediatric population
Introduction
A stepwise approach in the evaluation and interpretation of an electrocardiogram (ECG) is critical. Detailed instructions on a systematic approach to analyzing ECGs are beyond the scope of this chapter, but such an approach maximizes sensitivity and specificity.
Key Differences in Pediatric (electrocardiograms) ECG’s
In contrast to adult ECGs, pediatric ECGs exhibit key differences. Fortunately, the majority of these differences are predictable based on normal development and physiology. The neonatal heart is smaller (smaller volume chambers and lower muscular mass) and is more dependent on the right ventricle than the adult heart. The pulmonary vascular resistance is higher in-utero and drops as the child transitions from neonatal to adult physiology. Normal Pediatric ECG parameters will vary by age.
Smaller Heart:
Due to the smaller muscular size, electrical impulses travel more quickly, leading to shortened durations and intervals. Additionally, the heart rate is increased. Electrical durations and intervals seen on ECG will increase and the heart rate will decrease as the patient ages and the heart enlarges.
- Heart Rate: The neonatal heart rate is faster, as the small, noncompliant heart is dependent on heart rate rather than stroke volume to maintain cardiac output. The normal sinus rate decreases from 140 bpm at 1 month of age, to 120 bpm by 2 years of age, to 90 bpm by 10 years of age, and to 60-70 bpm for adolescents, approaching adult range.
- QT Interval: The QT interval is a reflection of the function of ion channels involved in repolarization and not myocardial thickness; it is most strongly modified by the HR. The baseline elevated pediatric heart rate increases the corrected QT interval (QTc).
- QTc = QTmeasured/HR
- Voltages: Neonatal voltages are overall smaller, and scale (the number of millimeters used to represent 1 mV) is often changed to optimize the ECG display.
- Impulses: The timing of electrical impulses is faster in the smaller heart. The PR interval is shorter in the neonate (~100 ms) and slowly increases to the adult level of ~160 ms by late adolescence. Similarly, the QRS duration is shorter in the neonate at ~50 ms and lengthens to the adult range of ~90 ms as the heart enlarges.
Greater Reliance on the Right Ventricle
The left ventricle (LV) in the adult is larger than the right ventricle (RV) and the LV thus accounts for the majority of the electrical forces. In the pediatric patient, the RV is relatively larger (see table), and is most dominant at 36 weeks of gestational age. The LV to RV ratio reaches adult proportions by 1-2 months of age. These dynamic changes lead to the changes in the morphology of ECG tracings as the patient ages.
| Age | LV to RV Weight Ratio |
| 30 weeks gestation | 1.2 : 1 |
| 36 weeks gestation | 0.8 : 1 |
| Birth | 1.5 : 1 |
| 2 months to adult | 2.5 : 1 |
- Right Axis Deviation: In the very young child (up to 1-2 months), there is rightward deviation (greater than +90 degrees) to the QRS axis in the frontal plane. This normalizes to around +60 degrees by approximately 6 months.
- Juvenile T waves: The T waves in leads V1-V3 are frequently negative or inverted in an infant, but the T waves in V2 and V3 usually become upright by the time that the child reaches adolescence. T waves are not normally inverted in V5 or V6. Many adult ECGs retain the inverted T wave in V1.
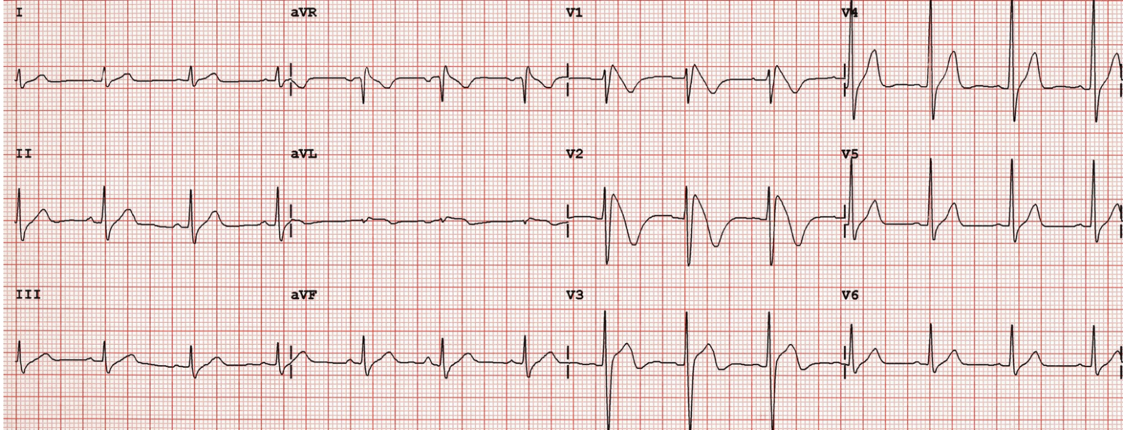
- Pseudo RBBB pattern: The neonatal RV has a greater muscular mass (and therefore a greater electrical presence on ECG) than the adult RV. This leads to a pseudo Right Bundle Branch Block (RBBB) pattern (RSR’ in the right precordial leads [V1-V3] with dominant R waves in the right precordium and dominant S waves in the left precordium) [image 1].
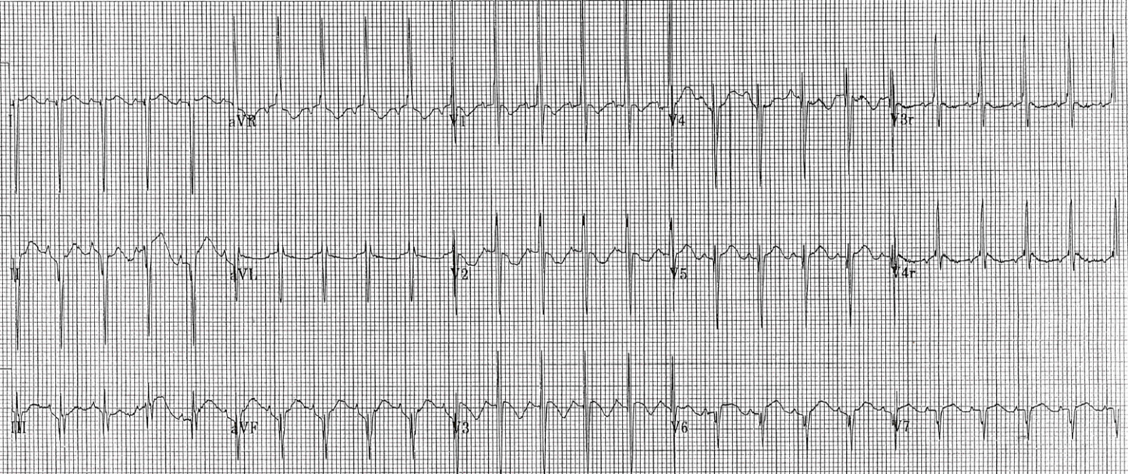
Pseudo Right Bundle Branch Pattern in Pediatric ECG
Common Pathology
Arrhythmias
Supraventricular Tachycardia
- In pediatric patients, supraventricular tachycardia (SVT) is a common tachydysrhythmia. Most causes of SVT are AV re-entry tachycardias (a cycle of electrical activity), which are distinct from ectopic atrial tachycardias (an abnormally fast firing source of electricity).
- SVT is recognized by narrow QRS, regular RR interval, absent or difficult to define P wave activity, and tachycardic rate. The baseline elevated HR in infants places the SVT rate significantly higher than in an adult and it is not uncommon to see SVT at a rate >220 bpm in a 1 month old child.
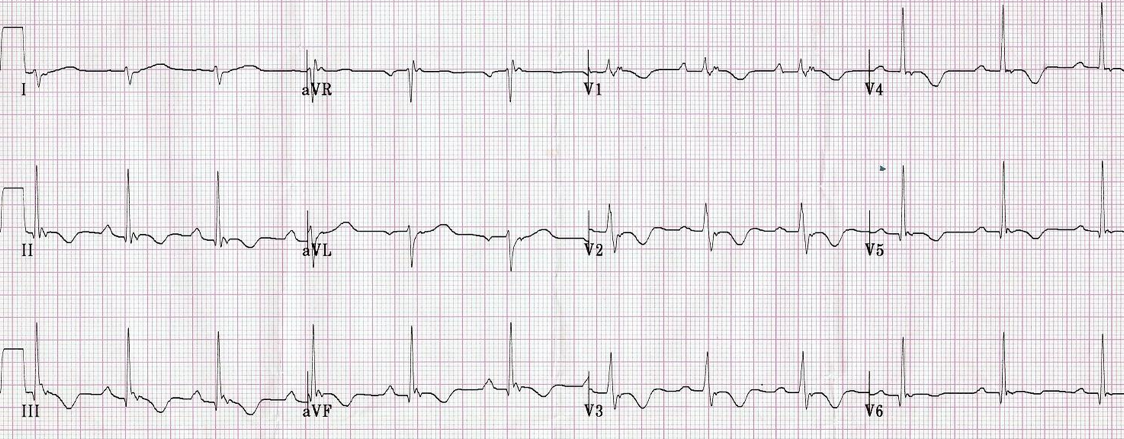
- Wolf Parkinson White (WPW). This is an accessory pathway that conducts between the atria and the ventricles. It can lead to pre-excitation (early partial depolarization of the ventricles), which manifests on an ECG as a slurred upslope at the beginning of the QRS complex (termed “Δ [delta]” wave), causing a shortened PR interval [image 2]. Keep in mind, the baseline shorter PR interval criteria in WPW are different in adults and children (the adult PR interval lower limit of normal is 120 ms, versus 75 ms for an infant).
- The accessory pathway may not become clinically apparent until the child presents with SVT. There are two subtypes of SVT in WPW:
- Orthodromic (“correct running”), wherein, the signal travelling down the AV node will distribute to the ventricles via the normal rapid conduction pathway and cause a narrow QRS. Then, the electrical signal will travel up the accessory/WPW pathway to re-excite the atria. This is the predominant pathway.
- Antidromic (“incorrect running”), wherein, the electrical signal travels down the WPW pathway, distributes via myocyte-myocyte conduction, and causes a slow ventricular depolarization (i.e. wide QRS). The electrical signal then travels back to the AV node to re-excite the atria.
- SVT can be treated with vagal maneuvers (e.g. dive reflex [application of ice packs to the face]) or adenosine. If the patient has evidence of significant hemodynamic instability, electrical synchronized cardioversion is used.
- SVT in WPW is treated the same as SVT in a normal heart (vagal maneuvers, adenosine, synchronized cardioversion).
- Verapamil (calcium channel blocker) should be avoided in infants < 1 year of age. It can cause accessory pathway acceleration in this age group.
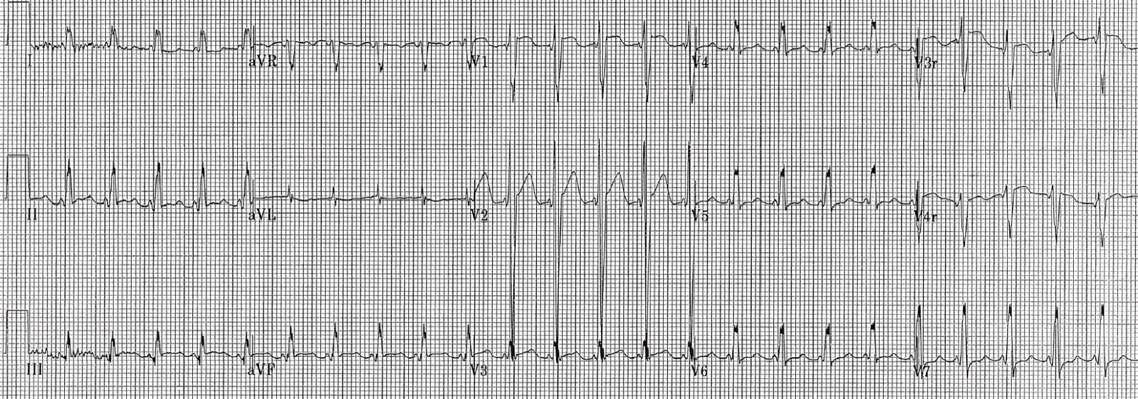
Shortened PR Interval as seen in Wolff-Parkinson-White
Heart block (AV block)
- Atrioventricular (AV) block is a disturbance in the conduction of electrical impulses from the atria to the ventricles. The same categories and treatment strategies that are applied in the adult population hold true in the pediatric population.
- First degree heart block: the PR interval is prolonged beyond the age appropriate level, but there are no ‘dropped beats’. This is asymptomatic and requires no treatment.
- Second degree heart block
- Mobitz I (Wenckebach) is characterized by progressive prolongation of the PR interval resulting in dropped beats. This likely requires repeat evaluation by PCP but not necessarily cardiology evaluation (see image below).
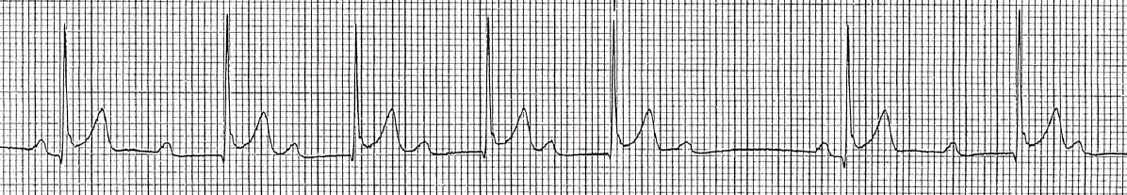
Second Degree Heart Block, Mobitz I Wenckebach
- Mobitz II is characterized by a fixed PR (quasi-normal or prolonged) with frequent dropped beats (often in a 3:1 or 4:1, conducted:dropped pattern). This has danger to devolve into complete heart block and warrants cardiology evaluation for potential pacemaker placement.
- Third degree/complete AV block: the atrial rhythm and the ventricular rhythm are completely dissociated. The PP interval is often regular, but does not correlate to the RR interval (which is often also regular). P waves are seen in every portion of the cardiac cycle (e.g. sometimes buried in the T waves, or in the QRS complex, or at varying places between the T and QRS). The QRS morphology is dependent upon the location of the ventricular pacemaker. For example, if the signal to fire the ventricles comes from the Bundle of His, the QRS will be narrow. But, if it arises from the left ventricle, the depolarization will travel slowly to the right ventricle, giving a wide QRS and a RBBB pattern. Third degree AV block requires evaluation by cardiology, for pacemaker placement.
Pathology Specific to Pediatrics
There are certain conditions in the pediatric population that can be identified on electrocardiogram.
Anomalous Left Coronary Artery [LCA] originating from the Pulmonary Artery (ALCAPA)
This describes a congenital cardiac abnormality in which the origin of the left coronary artery (LCA) is the pulmonary artery and not the aorta. This essentially causes myocardial ischemia in the territory of the LCA and can manifest post-partum with signs of heart failure.
Classically, prominent Q waves in the lateral leads (I, aVL, V3-V6) are seen on ECG.
Hypertrophic Cardiomyopathy (HCM)
This is a condition in which some of the heart is enlarged (usually the interventricular septum with or without some hypertrophy of the left ventricle). The hypertrophy of the left ventricle can cause arrhythmias both from the architectural distortion as well as from dynamic obstruction (where the hypertrophied segment can cause blood flow obstruction when the heart rate is high or preload is low, but might not manifest obstruction otherwise) of the left ventricular outflow tract. HCM must be considered in the young or adolescent patient who presents with syncope or near syncope, chest pain, or palpitations. Especially concerning historical features are the above symptoms in the setting of exertion, and a family history of unexplained or sudden cardiac death. These patients are treated with therapies that will enhance cardiac filling and preload in order to prevent malignant arrhythmias (e.g. heart rate slowing with beta blockade, exercise avoidance, ICD).
Due to the left ventricular hypertrophy, HCM classically presents on ECG with “dagger” q waves in the lateral leads (narrow, often < 40 ms in duration, and deep, best seen in V5-6 and I and aVL), large voltages across the precordium secondary to increased LV muscular mass, and nonspecific ST segment and T wave changes (see image below).
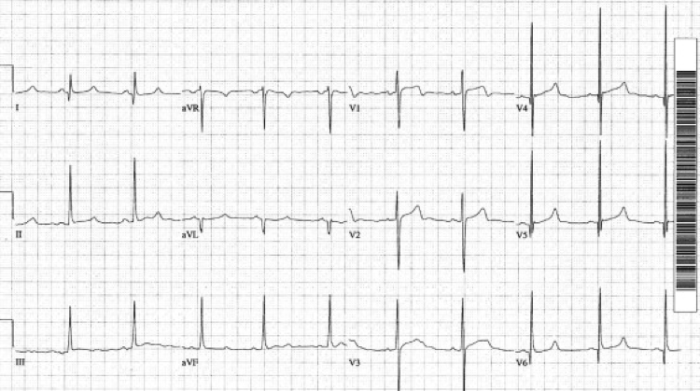
Hypertrophic Cardiomyopathy, note the dagger like q-waves in the lateral leads
Carditis
Inflammation of the myocardium can arise from both infectious causes (e.g. Coxsackie virus myocarditis) and inflammatory sources (e.g. Kawasaki disease). Patients with carditis can present with evidence of cardiogenic shock or new heart failure. The ECG in these patients can show nonspecific signs such as sinus tachycardia (most common) or display evidence classically associated with acute coronary syndromes such as ST Elevations, ST depressions, or T wave inversions [image 1].
Long QT
Abnormal repolarization of the heart can be caused by multiple genetic mutations or medications. This causes the T wave to separate from the QRS complex resulting in a long QT interval. Patients with long QT can be asymptomatic or may have a personal or family history of syncope or sudden cardiac death. Drugs such as macrolide antibiotics, ondansetron, antipsychotic agents, and phenothiazines also prolong the QT interval. If a subsequent ventricular depolarization stimulus (QRS complex) falls during a particularly sensitive part of the refractory period (R on T phenomenon), a ventricular arrhythmia known as torsades de pointes can arise (see image below).
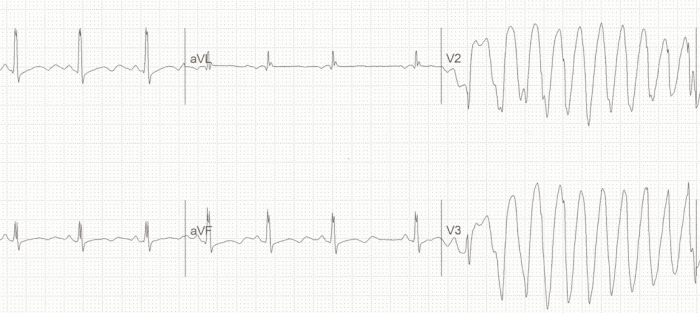
Long QT progressing into Torsade de Pointes
Brigade Syndrome
This is a common cause of otherwise unexplained sudden cardiac death. Brugada syndrome has multiple subtypes, but most are due to an abnormality in sodium channel function, leading to abnormal repolarization. Like long QT, it can lead to an ill-timed electrical impulse that can precipitate malignant arrhythmia, in this case ventricular fibrillation or ventricular tachycardia. Brugada Syndrome classically presents as syncope or sudden cardiac death. Brugada Syndrome is characterized by three separate morphologies (types 1, 2, and 3), with type 1 being most common. Type 1 manifests as a pseudo-RBBB appearance in the right sided precordial leads (e.g. V1, V2), with downsloping ST elevation leading to an inverted T wave (see image below). Brugada Syndrome is managed with placement of implantable cardioverter-defibrillator (ICD).
Brugada Syndrome
Arrhythmogenic Right Ventricular Dysplasia (ARVD)
This is a relatively common genetic condition wherein the myocardium, predominantly of the right ventricle, undergoes cell death and fatty degeneration leading to abnormal electrical signal conduction. This, too, can precipitate malignant arrhythmias, specifically ventricular fibrillation or ventricular tachycardia. The ECG presentation can be similar to Brugada Syndrome, in that the patient often has a RBBB with right precordial T wave inversion (TWI) (see image below). Unfortunately, while an ICD may be able to terminate arrhythmias, there is no current therapy to stop the disease progression.
Arrhythmogenic Right Ventricular Dysplasia (ARVD)
Classic Adult Pathology
Finally, in the appropriate clinical scenario, pathology that is classically thought of as limited to adults can present in pediatric patients and manifest on ECGs. For example, acute MI, T wave changes secondary to an intracranial process, and changes secondary to electrolyte abnormalities can all be seen on the ECG of a pediatric patient.
References
- Park and Guntheroth. “How to Read Pediatric ECGs.” Fourth Edition. 2006.
- D. Brekke et al. “Changing Electrocardiographic Patterns During Medical Treatment in a Patient With Anomalous Coronary Artery Originating From the Pulmonary Artery.” Circulation, April 24. 2001. Vol. 103. No. 16.
- ECG images from http://lifeinthefastlane.com, distributed under the Free and Open Access Medical Education (FOAMed) model.
- Kelly BS, Mattu A, Brady WJ. “Hypertrophic cardiomyopathy: electrocardiographic manifestations and other important considerations for the emergency physician.” Am J Emerg Med. 2007. Jan;25(1):72-9.
Approach to Pediatric ECGs
Authors: David Tillman, MD, Vanessa Tamas, MD, and Jamie Hess, MD
Editor: S. Margaret Paik, MD, Associate Professor of Pediatrics, The University of Chicago, Comer Children’s Hospital, Chicago, IL
Last Update: 2015
