Thoracic Aortic Dissection
Author: Matthew P. Borloz, MD, Virginia Tech / Carilion School of Medicine, Carilion Clinic Department of Emergency Medicine
Editor: Tim Curlett MD
Last Updated: 2019
Case Study
56-year-old woman with a history of hypertension presents with abrupt onset of “band-like” chest and back pain, worse with supine position and deep inspiration. Onset was 2 hours prior while seated on the couch. Pain is moderate to severe. She has no history of similar pain, no history of coronary disease, and no family history of interest. She denies alcohol, tobacco, or illicit drug use and had no neurologic symptoms. Physical exam reveals an aortic insufficiency murmur, which is not known to be chronic. Neurologic exam is normal, including motor, sensory, and cranial nerve testing. 12-lead ECG shows sinus tachycardia with normal axis, normal intervals, and no ischemic changes.
Objectives
Upon completion of this module, the student should be able to perform the following:
- Describe the Stanford classification scheme for aortic dissection
- List at least three risk factors for acute aortic dissection
- Detail at least two benefits and two drawbacks of each of the following imaging modalities, as they relate to the diagnosis of thoracic aortic dissection: CXR, CTA, MRI, TEE, and TTE
- Explain how the emergent management of Stanford type A dissection differs from that of Stanford type B dissection
- Describe the features of a “complicated” Stanford type B dissection.
Introduction
Thoracic aortic dissection should be considered for every patient presenting to the emergency department with chest pain or back pain, particularly if accompanied by neurologic signs or symptoms. Uncommon and difficult to diagnose, this condition is associated with serious, often lethal, complications. Prompt diagnosis and emergent surgical consultation are vital to achieve a favorable outcome, though sometimes even this is insufficient. Half of patients with a type A dissection die within 24-48 hours without appropriate treatment, and even with surgical management, mortality may exceed 25%. According to recent data from a large international registry, overall in-hospital mortality rates for patients who arrived to centers specialized in the treatment of this condition were 22% for type A dissection and 13% for type B dissection.
Alternative diagnoses on the differential include the following life-threatening conditions: acute coronary syndrome, aortic aneurysm, cardiac tamponade (from another cause), esophageal rupture (Boerhaave syndrome), pneumonia, pneumothorax, pulmonary embolism, and stroke / transient ischemic attack.
Epidemiology & Risk Factors
The incidence of thoracic aortic dissection is approximately three per 100,000 person-years. Because of the high mortality and elusive nature of this disease, this is likely a gross underestimation. Many patients likely die before reaching the hospital, and in the absence of an autopsy, their cause of death may be attributed to more common conditions such as acute MI.
Thoracic aortic dissection is at least twice as common in males as females, and the prevalence of this condition increases with age. Risk factors are numerous and include genetic syndromes and both acute and chronic cardiovascular conditions. Genetic syndromes that place patients at increased risk of thoracic aortic dissection include Marfan syndrome, Loeys-Dietz syndrome, Turner syndrome, and Ehlers-Danlos syndrome (vascular type). Patients with a family history of aortic dissection or aneurysm, or with a personal history of aortic aneurysm or coarctation, chronic hypertension, acute hypertension (as in stimulant abuse), polycystic kidney disease, inflammatory vasculitis (e.g., giant cell arteritis), or pre-existing aortic valvular disease (e.g., bicuspid aortic valve), are also at increased risk. Recent aortic manipulation (open or endovascular) should raise one’s pre-test concern for this condition, and pregnancy elevates the risk for women with chronic connective tissue disorders. Finally, patients with long-term exposure to corticosteroids or other immunosuppressive drugs are at increased danger of developing aortic dissection.
Classification & Terminology
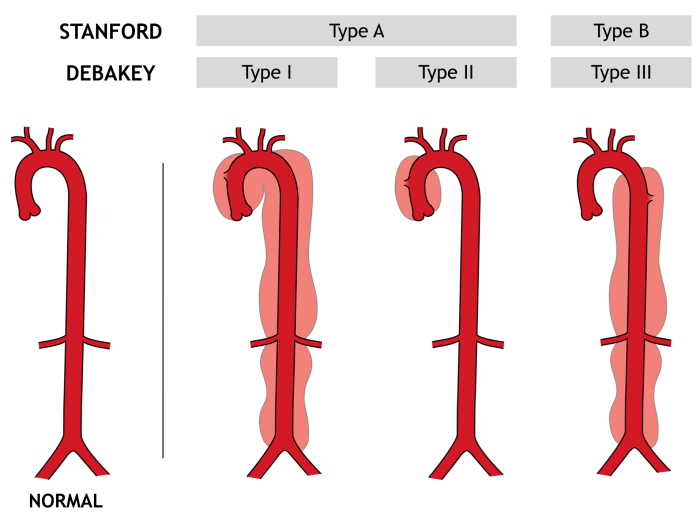
Figure 1. Aortic dissection classification. The Stanford classification is more commonly used. Type A dissections involve the ascending aorta, while type B dissections do not. The DeBakey classification further separates Stanford type A dissections into types I and II, depending on whether the arch and descending aorta are involved. Original figure M. Borloz. Used by permission. Content provided by CC BY-NC-SA. No changes have been made. 2019
Two principal classification schemes exist to describe the portion of the aorta affected by the dissection (Figure 1). The Stanford classification is most often used in emergency medicine practice, but the DeBakey system will be briefly introduced, as well. Simply put, Stanford type A dissections involve the ascending aorta, while Stanford type B dissections do not. Type A dissections may also affect the descending aorta, but because ascending aortic involvement is generally the more important question, this is the sole determinant of the Stanford type. Briefly, DeBakey type I affects both the ascending and descending aorta, as well as the aortic arch; type II affects only the ascending aorta; and type III affects only the descending aorta. Stanford type A overlaps with DeBakey types I and II, while all Stanford type B dissections are DeBakey type III. Approximately two-thirds of aortic dissections are classified as type A.
Aortic dissection is generally considered acute if less than 14 days have elapsed since the dissection occurred and chronic if >90 days have passed, though determining exact time of onset can be difficult, and authors differ with respect to definitions of hyperacute, acute, subacute, and chronic.
Sometimes, the term “acute aortic syndrome” is used to include classic aortic dissection, as well as intramural hematoma and penetrating aortic ulcer. The management of the latter two is less clear and should be guided primarily by consultation with a cardiothoracic surgeon. The discussion herein will focus only on true aortic dissection, in which a tear in the aortic intima permits entry of blood into the media (Figure 2). Pulsatile flow into the rent created by the tear propagates proximally and/or distally along the length of the aorta. This may disrupt flow at the origin of any of the branch vessels, or the dissection may continue along the branch vessels to disrupt flow more distally. This includes any branches off the aorta, from the coronaries all the way down to the iliacs. When considering the presentation and morbidity of aortic dissection, one may imagine the complications that would result from occlusion of any of these branches. Should the blood penetrate the adventitia, leak or frank rupture occurs.

Figure 2. Cross section through the aorta. Blood “dissects” into the media through an intimal tear at a given level and propagates proximally and/or distally. Signs, symptoms, morbidity, and mortality all depend on the level of the dissection and the branch vessels involved. Original figure by M. Borloz. Content provided by CC BY-NC-SA. No changes have been made. 2019
Initial Actions and Primary Survey
Summary of actions for unstable patients in whom thoracic aortic dissection is highly suspected based on initial history and focused exam:
- ABCs
- 2 large-bore IVs
- Uncrossmatched blood to the bedside
- Cardiorespiratory monitoring
- 12-lead ECG
- Portable CXR
- Bedside cardiac ultrasound for pericardial effusion & systolic function
- Labs – CBC, CMP, PT/PTT, type & cross, troponin, lactate
- Page cardiothoracic surgeon
For patients in whom you are strongly considering a diagnosis of thoracic aortic dissection, several actions should be pursued. As for all patients presenting to the emergency department, formation of a general impression of a patient’s status (i.e., “sick or not sick”) upon entering the room is followed by a rapid assessment and immediate management of the patient’s airway and breathing. Ill-appearing patients should elicit concern and prompt you to summon additional resources to perform interventions while you simultaneously proceed with your assessment. Establishment of two large-bore (i.e., 18-gauge or larger) intravenous lines, as well as placement of cardiac and respiratory monitoring equipment, should be pursued at once. Defibrillation capability should be close at hand, though its likely benefit may be limited (for patients who have arrested due to a complication of aortic dissection).
Following establishment of acceptable airway and breathing conditions, attention should be aimed at a thorough assessment of the patient’s circulation. Patients exhibiting hypotension and shock in whom you highly suspect thoracic aortic dissection should be administered uncrossmatched blood in the absence of contraindications. The presence and strength of pulses in all four extremities should be determined, and this should be accompanied by measurement of bilateral upper extremity blood pressures with increased concern for thoracic aortic dissection when a systolic BP differential of greater than 20 mmHg between arms is obtained.
Auscultation of an aortic insufficiency murmur should similarly raise your suspicion for type A dissection, as distortion or dilatation of the aortic root leads to impaired coaptation of the valve leaflets.
Because the majority of patients with thoracic aortic dissection will present with chest pain, a 12-lead electrocardiogram should be requested, as should chest radiography. Until patient stability is ensured, portable chest x-ray may be most appropriate, though this will neither serve to rule in nor rule out the diagnosis. The presence of a widened mediastinum or aorta is suggestive of the diagnosis, and findings such as an apical pleural cap or displaced intimal calcification are consistent with the diagnosis, though none of these abnormalities is reliably present (see below). Advanced diagnostic imaging can be obtained subsequently if the patient demonstrates a stable initial course.
Particular caution is advised at this stage, as a 12-lead ECG diagnostic of an ST-elevation myocardial infarction does not exclude concomitant aortic dissection. A type A dissection may indeed be the cause of the STEMI. Certain therapies pursued to address the STEMI, in the absence of definitive surgical management of the ascending aortic dissection, may render harm to the patient. Imagine the consequence of thrombolytic therapy administered to a patient with a hemorrhagic pericardial effusion from their aortic dissection. Having said this, the incidence of STEMI dwarfs that of acute aortic dissection, so delays in treatment to absolutely exclude the latter in every case would likely result in a net increase in overall population morbidity and mortality. This discussion is included only to prompt consideration of acute aortic dissection when you diagnose STEMI, not to mandate an exhaustive workup to exclude it in all patients. Approximately 5% of patients with type A dissection, in one large series, showed concomitant electrocardiographically evident MI.
Vital Signs
Blood pressure is often elevated, especially in patients with type B dissection, though a normal or hypotensive reading may indicate complications of the dissection, such as retroperitoneal hemorrhage or cardiogenic shock from myocardial ischemia or acute aortic insufficiency. Heart rate is commonly elevated as well, likely due to excruciating pain and anxiety, though tachycardia may also be compensatory in the case of cardiac tamponade or internal hemorrhage. Bradycardia (i.e., sinus bradycardia or varying degrees of atrioventricular block) may be seen in the setting of inferior or anterior MI that results from proximal aortic dissection involving the coronary arteries. Increased respiratory rate is expected, largely due to pain and anxiety, though it may also result from a compensatory effort to correct metabolic acidosis from intestinal, renal, or other tissue malperfusion. Hemorrhagic pleural effusions may impair oxygenation and ventilation and drive the respiratory rate up and the oxygen saturation down.
Presentation
History
The classic patient is a male in his 60s with a history of chronic hypertension who presents with “sharp,” sudden-onset, severe chest pain that radiates to the back.
Three-quarters of patients presenting with acute thoracic aortic dissection will complain of chest pain, and greater than 95% will complain of pain in some location in the back. Classically, this pain is sudden-onset and may radiate to the interscapular area of the back (i.e., “between the shoulder blades”) or to the abdomen and low back, depending on the location of the dissection. The pain is typically the most severe the patient has experienced and may be described as “sharp,” “ripping,” or “tearing” in quality.
Approximately one out of every six patients will exhibit neurologic symptoms. Syncope is reported in roughly 15% of cases and may be due to a number of causes (e.g., cardiogenic shock from tamponade or myocardial ischemia, hemorrhagic shock from rupture, bilateral carotid occlusion).
Physical Exam
Less than half of all patients with aortic dissection will display physical exam abnormalities historically described for this condition. Patients will exhibit a peripheral pulse deficit in 15-30% of cases, approximately two-fold more commonly in type A dissection as compared to type B dissection. A diastolic murmur is identified in slightly less than 30% of cases. Stroke is diagnosed in approximately 5% of cases, and typical neurologic deficits consistent with stroke may be identified on exam. There are, unfortunately, no features of the history or physical exam that are pathognomonic for aortic dissection, nor are there any that reliably exclude it.
Differences in Presentation by Stanford Type
Patients with type A dissection often present slightly differently than those with type B dissection. Type A dissection is more likely to cause chest pain or syncope and less likely to cause back or abdominal pain when compared to type B dissection. Patients with type B dissection are about two times as likely to present with hypertension (SBP ≥150 mmHg), whereas type A dissections are many times more likely to cause hypotension (SBP<100 mmHg) with or without frank shock.
Diagnostic Testing
A high index of suspicion must be maintained for this diagnosis given its substantial morbidity and mortality. In one series, over one-third of patients were misdiagnosed on their first presentation to the emergency department. There are no widely accepted clinical decision rules that are adequately sensitive for ruling out a diagnosis of thoracic aortic dissection. Conditions with such a high mortality and such a low incidence make development of a clinical decision rule exceedingly difficult.
Diagnostic Imaging
As with all undifferentiated patients in the emergency department, arrival at a definitive diagnosis so that appropriate condition-specific treatment may be rendered is the goal. Diagnostic imaging plays a substantial role in meeting this objective in the case of thoracic aortic dissection. A variety of imaging modalities are available in the emergency department, though CT angiography is the most widely used definitive study for this condition. Additional options that share excellent sensitivity and specificity include MRI and transesophageal echocardiography, but each has its drawbacks. The available modalities will be discussed in turn.
Chest X-Ray (CXR)
Chest x-ray is widely available, inexpensive, rapidly acquired and processed, and obtained without the need for sedation, transport from the department, IV access, or supine positioning. In addition, patients are exposed to a minimal amount of radiation. Unfortunately, ~15% of patients with confirmed aortic dissection will lack abnormal CXR findings to suggest it, so this study alone is insufficient to exclude the diagnosis. Findings that should further raise your concern for thoracic aortic dissection include the following:
- Widened mediastinum (>8 cm at aortic knob)
- Abnormal aortic or cardiac contour
- Displaced intimal calcification
- Widened right paratracheal stripe (≥5 mm)
- Tracheal deviation (usually rightward)
- Opacified aortopulmonary window
- Pleural effusion (usually left)
CT Angiography (CTA)
In stable patients, CTA is the most commonly employed imaging study used for ruling aortic dissection in or out (Figure 3). It has excellent sensitivity and specificity, each approaching 100% with newer machines and techniques. In addition, CTA provides an alternative diagnosis in one-fifth of patients imaged for investigation of acute aortic dissection; this is particularly important in the setting of such an uncommon condition. Because end-organ perfusion is a concern in patients with acute aortic dissection, the ability of this modality to directly assess for compromised perfusion to the gut, kidneys, brain, or lower extremities is advantageous. CTA demonstrates differential enhancement for identification of true and false lumina, and three-dimensional reconstructions provide information crucial for operative planning. Images should be obtained from the lung apices through the iliacs, so that all of the branch vessels off the aorta are included.
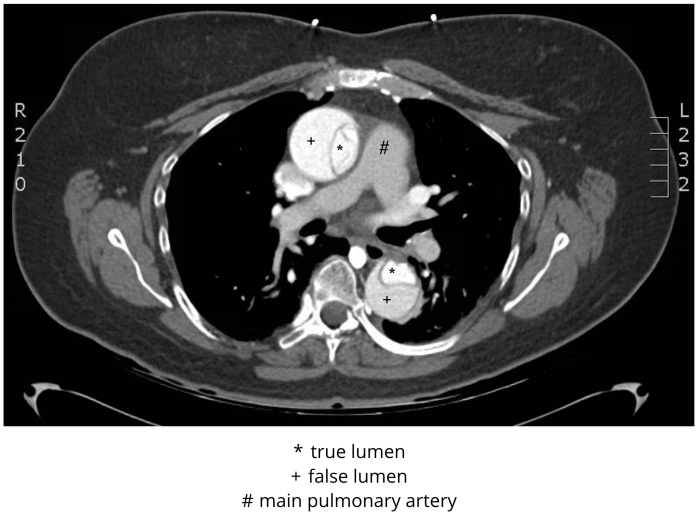
Figure 3 CT angiogram, Stanford type A (DeBakey type I) dissection. De-identified CT angiogram with labels applied by M. Borloz. Original image. Content provided by CC BY-NC-SA. No changes have been made. 2019
The disadvantages of CTA are few but may be important in certain cases. Radiation exposure is significant, and efforts should be made to limit unnecessary exposure; however, this truly is the most appropriate test for the majority of patients in the emergency department in whom you have concern for this diagnosis. As CTA requires IV contrast administration, individuals with contrast allergy or renal insufficiency (acute or chronic) may pose a challenge, but the risks of delayed diagnosis of aortic dissection (to summon consultants and equipment to the bedside for TEE) must be weighed against the risks of a true allergy or contrast-induced nephropathy. Protocols for these situations vary by institution. Another limitation is that patients have to leave the emergency department to have the study done, and if they decompensate, recognition and appropriate intervention may be delayed as a consequence of this. In addition, patients have to lie supine for this study, and if pain, anxiety, or respiratory difficulty limit their ability to do so comfortably, the study may be unobtainable or limited by motion artifact. Finally, CTA is a “static” study in that it gives no definitive information regarding the presence of aortic valvular dysfunction.
Magnetic Resonance Imaging (MRI)
While MRI has excellent test characteristics for diagnosis or exclusion of this condition, it is almost never an appropriate test to diagnose acute aortic dissection in the emergency department. In the majority of hospitals, the physical distance between the MRI magnet and the emergency department is too great to be safe for patient care. In addition, MRI studies take longer to obtain. Finally, issues with incompatible metal implants or implanted biomedical devices (e.g., pacemaker) may limit which patients can safely approach the magnet. As an emergency department imaging modality for this indication, MRI should be used only as a last resort, in select cases among absolutely stable patients and with the direct input of your cardiothoracic surgery consultant. As a means of following repaired or chronic aortic dissections, this is an excellent option.
Transesophageal echocardiography (TEE)
The primary benefits of TEE are that it allows for bedside diagnosis of this condition in unstable patients who should not leave the department to obtain an imaging study. The sensitivity and specificity closely mirror those of CTA in capable hands, and the patient need not be exposed to radiation or potentially nephrotoxic iodinated contrast agents. Finally, TEE is a dynamic test that allows for assessment of aortic valve structure and function, as well as the presence or absence of an intimal flap in the aortic root, arch, and descending thoracic aorta. Questions about aortic insufficiency can be answered directly. This procedure does not necessarily require endotracheal intubation, though one should appreciate that the anxiety associated with the procedure in an awake patient may complicate heart rate and blood pressure management.
Transthoracic echocardiography (TTE)
With the increasing penetration of bedside ultrasonography into emergency department practice, this is a tool that is readily available to answer some critical questions in an unstable patient with an aortic dissection. While it is poorly sensitive for diagnosis of type B dissection and only moderately sensitive for type A dissection (in expert hands), it is often helpful in demonstrating the presence or absence of a pericardial effusion or wall motion abnormalities. Aortic insufficiency may also be demonstrated with this modality, though this is not a finding most emergency physicians are accustomed to identifying. The aortic root and descending thoracic aorta can often be partially visualized with TTE, though the arch is not easily seen in adults.
Electrocardiogram (ECG)
As the majority of these patients will present with chest pain and/or hemodynamic instability, all should have a 12-lead ECG. Nearly one-third will have a normal tracing, though approximately 5% of patients with type A dissection will have an ECG diagnostic of acute MI. The acute MI may be a direct consequence of a dissection into the coronary ostia. The right coronary artery is more commonly affected than the left, so inferior changes are more likely. Ischemia without infarction is identified in 15% of cases. Left ventricular hypertrophy is seen in one-quarter of patients with aortic dissection, but this is such a common finding that its presence is not helpful for diagnosis.
Lab testing
The only lab test that has been reasonably studied and shows any promise as a screening tool for thoracic aortic dissection is D-dimer; however, because of its lack of specificity, ordering this assay may result in an increase in the number of imaging studies done to exclude the diagnosis. In addition, false negative results can be seen in a number of scenarios, such as a thrombosed false lumen or chronic aortic dissection. D-dimer is not currently recommended as a screening test for thoracic aortic dissection, though recent data looking at combined use of the Aortic Dissection Detection Risk Score and D-dimer may show promise. Other lab tests that may be ordered for these patients, and their expected findings, include the following:
- Hemoglobin – low from internal hemorrhage into an extravascular space, such as pleural or retroperitoneal (remember that acute or minor hemorrhage may not be reflected in the hemoglobin immediately after the hemorrhage occurs)
- White blood cell count – elevated from stress response to an aortic catastrophe or intestinal malperfusion
- Lactic acid – elevated from intestinal malperfusion
- Creatinine – elevated from renal malperfusion
- Troponin – elevated from coronary artery dissection with myocardial necrosis
None of these lab tests are sufficiently sensitive to exclude the diagnosis of thoracic aortic dissection, and none is specific enough to establish the diagnosis. They do, however, provide ancillary information that is helpful in further defining the morbidity associated with the diagnosis, once made, and determining the appropriate course of treatment.
Treatment
Treatment varies substantially based on Stanford classification. Type A dissections require emergent surgical repair, while type B dissections are largely managed medically in the acute phase. “Complicated” type B dissections (see below), which occur in 20-30% of patients, may be managed medically or by open surgical or endovascular techniques, depending on a variety of features to be carefully weighed by the patient and surgeon.
Addressing the terrible pain associated with acute aortic dissection is crucial. Not only is this ethically appropriate, it will help to control the patient’s heart rate and blood pressure. Additionally, the patient should be kept NPO, so as to minimize airway complications should they suddenly deteriorate in the ED or require an immediate trip to the OR.
For the emergency department management of both type A and type B dissections, keeping both the heart rate and blood pressure as low as possible while still ensuring end-organ perfusion is the objective. Limited evidence exists to support specific blood pressure goals; however, the 2010 ACC/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease recommend the use of beta-adrenergic blockers (or calcium-channel blockers if contraindications to beta-blockers exist) to reduce the heart rate to 60 beats per minute or less. If a systolic blood pressure greater than 120 mmHg persists after use of these initial agents, they direct the addition of intravenous vasodilators to lower the blood pressure further. Again, end-organ perfusion should not be compromised in the interest of targeting a specific number.
In order to help visualize these goals, imagine a tiny wedge positioned within the wall of the aorta so that advancing the wedge would propagate the dissection. Now imagine that each heartbeat is a hammer strike on the end of the wedge, and the power with which the hammer strikes the wedge is determined by the blood pressure. You can appreciate why lowering both the heart rate and the blood pressure is important. Fifty hammer strikes per minute is better than 80, and a weak strike is better than a strong one.
There is no single agent that is universally recommended over another, though the short-acting and titratable characteristics of the beta-adrenergic blocker, esmolol, make it a theoretically ideal agent for initial rate control. As for vasodilator selection, sodium nitroprusside has classically been used, though nicardipine, a vascular-selective calcium channel blocker, has been shown to be safe and effective in this population and is preferred by many clinicians.
Though the majority of type B aortic dissections do not require immediate surgical management, certain features render them “complicated”. When end-organ perfusion is compromised (e.g., intestinal ischemia, spinal cord ischemia, renal malperfusion), hypertension is refractory to medications, the dissection continues to propagate despite medical interventions, or the aorta exhibits aneurysmal expansion, open surgical or endovascular procedures may be indicated. Sometimes these conditions are evident at the time of emergency department presentation; alternatively, these complications can develop years after the initial dissection or at any time in between. Among patients taken for open surgical intervention, mortality approaches one-third; however, more recent experience with endovascular techniques has reduced mortality to as low as 5%.
Pearls and Pitfalls
STEMI on ECG does not exclude concomitant aortic dissection and may be a consequence of it. Do not give thrombolytics before you have considered and reasonably excluded aortic dissection (i.e., no H&P features or CXR findings to suggest the diagnosis).
When giving thrombolytics in the case of STEMI, document the medical reasoning used to support your low suspicion for aortic dissection (e.g., normal mediastinum on CXR, no upper extremity BP differential, equal pulses in all extremities, no neurologic findings, no murmur, gradual onset of pain, absence of back pain).
Always take the patient’s stability into consideration when determining the most appropriate diagnostic imaging modality. Unstable patients should not leave the department for diagnostic testing.
D-dimer is not adequately sensitive for aortic dissection to independently exclude the diagnosis.
Heart rate and blood pressure should be kept as low as possible while maintaining end-organ perfusion.
Vasodilators should not be initiated prior to rate-controlling agents, as they may result in reflex tachycardia that serves to propagate the dissection.
Pitfall: failure to obtain immediate surgical consultation for all patients with aortic dissection, even for patients with type B dissections or those presumed to be chronic. Let the surgeon decide that the patient does not need emergent surgery.
Pitfall: failure to promptly manage heart rate and blood pressure, even in patients destined for the operating suite.
Pitfall: ruling out the diagnosis based on false-negative results of insensitive diagnostic tests or physical exam findings.
Case Resolution
Case Resolution: D-dimer was elevated at 17 mcg/mL (FEU). CTA chest confirmed aortic dissection. The patient underwent emergent graft replacement of the aortic root and ascending aorta with single-vessel aortocoronary bypass grafting. She subsequently underwent delayed endovascular repair of the descending thoracic component.
References
- Ankel FK, Stanfield SC. Aortic dissection. In: Walls RM, Hockberger RS, Gausche-Hill M, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Philadelphia, PA: Elsevier; 2018. https://www.clinicalkey.com/#!/browse/book/3-s2.0-C20141019850. Accessed March 21, 2019.
- Baliga RR, Nienaber CA, Bossone E, Oh JK, Isselbacher EM, Sechtem U, et al. The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging. 2014;7(4):406-24. PMID: 24742892
- Broder JS. Diagnostic imaging for the emergency physician. Philadelphia, PA: Elsevier; 2011. Chapter 7, Imaging of pulmonary embolism and nontraumatic aortic pathology; p373-443.
- Clouse WD, Hallett JW, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. May Clin Proc. 2004;79(2):176-80. PMID: 14959911
- Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, Trimarchi S, Braverman AC, Myrmel T, Harris KM, Hutchinson S, O'Gara P, Suzuki T, Nienaber CA, Eagle KA; IRAD Investigators. nsights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation. 2018;137(17):1846-60. PMID: 29685932
- Fattori R, Cao P, De Rango P, Czerny M, Evangelista A, Nienaber C, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61(16):1661-78. PMID: 23500232
- Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897-903. PMID: 10685714
- Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55(14):e27-129. PMID: 20359588
- Johnson GA, Prince LA. Aortic dissection and related aortic syndromes. In: Tintinalli JE, Stapczynski J, Ma O, Yealy DM, Meckler GD, Cline DM, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016. http://accessemergencymedicine.mhmedical.com/content.aspx?bookid=1658§ionid=109429195. Accessed March 21, 2019.
- Karthikesalingam A, Holt PJE, Hinchliffe RJ, Thompson MM, Loftus IM. The diagnosis and management of aortic dissection. Vasc Endovascular Surg. 2010;44(3):165-9. PMID: 20308170
- Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA. 2002;287(17):2262-72. PMID: 11980527
- Mery CM, Reece T, Kron IL. Chapter 50. Aortic Dissection. In: Cohn LH. ed. Cardiac Surgery in the Adult, 4e. New York, NY: McGraw-Hill; 2012. http://accesssurgery.mhmedical.com.proxy1.athensams.net/content.aspx?bookid=476&Sectionid=39679067. Accessed September 28, 2014.
- Nazerian P, Mueller C, Soeiro AM, Leidel BA, Salvadeo SAT, Giachino F, et al. Diagnostic accuracy of the Aortic Dissection Detection Risk Score plus D-dimer for acute aortic syndromes: the ADvISED prospective multicenter study. Circulation. 2018;137(3):250-8. PMID: 29030346
- Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23. PMID: 23109648
- Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012;33(1):26-35. PMID: 21810861
- Reed KC, Curtis LA. Aortic emergencies: part I – thoracic dissections and aneurysms. Emerg Med Practice. 2006;8(2):1-24.
- Teece S, Hogg K. Best evidence topic report. Peripheral pulses to exclude thoracic aortic dissection. Emerg Med J. 2004;21(5):589. PMID: 15333542
- Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg. 2009;37(2):149-59. PMID: 19097813
- von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160(19):2977-82. PMID: 11041906
