Pulmonary Embolus
Home
/
About SAEM
/
Academies, Interest Groups, & Affiliates
/
CDEM
/
For Students
/
CDEM Curriculum
/
M4 Curriculum
/
Pulmonary Embolus
Author: Edward Bruno, MD, Christopher Fowler, DO, University of Arkansas for Medical Sciences, Little Rock, Arkansas
Editor: Robert J Hyde, MD MA, Mayo Clinic- Rochester MN
Last Updated: November 2019
Case Study
Patient is a 56 yo male with past medical history of hypertension and type 2 diabetes who presents with a 3 day history of progressively worsening shortness of breath and mild chest pain. He reports that the shortness of breath was sudden in onset and is worsened with exertion. He also complains of a tightness in his chest that is worse with deep inspiration. Denies additional symptoms. Denies recent surgery but does endorse a recent trip to Europe to visit family. Denies fever, abdominal pain, nausea or vomiting or hemoptysis. Endorses a mild, non-productive, dry cough when he feels short of breath.
On exam the patient appears uncomfortable but non-toxic. He has a mildly increased work of breathing. VS are BP 145/92, HR 105, RR 22, SpO2 91% on RA. Breath sounds are clear bilaterally and hear ratet is tachycardic but has a normal rhythm. Distal pulses
are intact. Trace bilateral pedal edema.
Objectives
- Discuss the signs and symptoms classic for PE & DVT
- Discuss non-classic presentation of PE
- Discuss the utility of clinical decision rules in developing a pre-test probability for the workup of DVT/PE
- Discuss the use of the D-dimer in the diagnosis of pulmonary embolus and DVT and understand age-related cutoff
- Compare imaging modalities used in the workup of patients with suspected thromboembolic disease.
- Understand the treatment options and ED dispositions for patients with thromboembolic disease.
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) together comprise a disease process called venous thromboembolism (VTE). A relatively common diagnosis with approximately 200,000 patients being diagnosed with new or recurrent PE each year, VTE may often be deadly, and as such should not be missed during diagnostic workup in the emergency department (ED). In fact, PE is the second leading cause of unexpected death found during autopsy. DVT/PE are generated from thrombus, typically in the lower extremity vessels related to venous stasis or other hypercoagulable states. In PE, thrombus is dislodged from the deep vessels and is translocated to the lungs. The ventilation/perfusion mismatch is typically what causes the symptoms of shortness of breath and tachycardia.
Unfortunately, the presentation of DVT/PE can be non-specific and mimic other disease processes.
VTE can be difficult to diagnose as patients can present with atypical symptoms and presentation; therefore, it is important to have a high index of suspicion and to understand the workup in ruling out this disease process in our ED
patients.
Initial Actions and Primary Survey
As with all patients presenting to the Emergency Department, an assessment of the ABC’s is critical. Occasionally patients with PE will present in distress, but more often there are subtle signs and symptoms that must raise suspicion of PE. In patients with a large clot or saddle emboli, the presenting symptoms can be cardiopulmonary arrest necessitating the need for advanced measures to manage the patients ABC’s.
Isolated DVT does not typically result in severe morbidity or mortality. One concern with DVT is extension and embolization to the lungs.. PE may present as mild shortness of breath, chest pain, fatigue, or a number of other non-specific symptoms.
As with other patients complaining of chest pain or shortness of breath, those with suspected PE should be placed on a cardiac monitor, have IV access established, and supplied supplemental oxygen as needed.
An initial EKG and CXR should be obtained in patients with suspected PE to rapidly evaluate for other items on the differential diagnosis (MI, pneumonia, pneumothorax).
Presentation
Classic Presentation of Deep Venous Thrombosis
Initial DVT symptoms may be subtle and nonspecific. Complaints include general leg pain or a cramping sensation, fullness in the calf, swelling, edema or tenderness on palpation.
The differential diagnosis may include musculoskeletal strain or tear, cellulitis, superficial thrombophlebitis, venous insufficiency, lymphedema, or popliteal (Baker’s) cyst. Signs or symptoms of the above diagnoses are therefore important to note as pertinent positive and negatives during your physical exam and documentation.
Classic physical examination findings of DVT include unilateral swelling or edema of the extremity, tenderness to palpation, and a palpable venous “cord.” Homan’s sign is the classic sign of pain in the calf on passive dorsiflexion of the foot with the knee in extension. This test is neither sensitive nor specific in ruling in or ruling out DVT of the extremity.
Your initial history and survey should include all the pertinent information to develop a differential and calculate a pretest probability. Risk factors may help estimate the risk of venous thromboembolism in patients and calculate a pretest probability. The Wells score is a clinical decision rule developed to calculate the pretest probability of DVT as well as PE, and to help guide further diagnostic workup. Again, a high index of suspicion is warranted for patients with concerning history and physical exam findings
Please click on the link to calculate a Pre-Test Probability (Wells Score) for DVT.
Classic Presentation of Pulmonary Embolus
The classic presentation of PE includes complaints of shortness of breath and/or chest pain. Additionally, syncope, as well as vague complaints of general malaise or functional deterioration, may be presenting features. While often vague, patients may describe a pleuritic component to the chest pain (i.e. hurts worse with deep breaths). Unilateral leg symptoms (DVT symptoms-see above) may be present, as well as signs of right sided heart failure (jugular venous distention, peripheral edema). The most common vital sign abnormality seen is tachycardia in the setting of normal pulse oxygenation. It is important to note that patients may have no respiratory complaints, making this diagnosis quite difficult to identify.
Physical exam findings typically include tachycardia, dyspnea, hypoxia and possibly mildly elevated temperature. The degree of dyspnea is not associated with findings on pulmonary exam, which can be clear and without abnormality. Cardiac exam may
be normal or can occasionally have S3 sound. The most common abnormal vital sign in PE is tachycardia.
Risk Stratification
Clinical decision rules developed for risk stratification of suspected PE include Wells Criteria for PE as well as the Pulmonary Embolism Rule Out Criteria ( PERC). These rules can help guide diagnostic testing. Well’s uses clinical features to determine low, moderate, or high-risk patients.
In patients who are low risk according to Wells, the PERC criteria may be applied. If a patient is low risk according to Wells and meets all of the PERC criteria, they are considered very low risk for PE and would not benefit from—and may actually be harmed by—further testing.
Patients who are moderate risk for PE according to Wells should undergo d-dimer testing. In most patients the threshold for a positive d-dimer test is 500 ng/mL. Normal d-dimer values increase with age, so 100 ng/mL may be added per decade of life over the age of 50: E.g. the threshold for a positive test is 600 ng/mL in a 60 year old, 700 ng/mL in a 70 year old, etc.
High risk patients according to Wells criteria for PE require imaging with either CT angiography of the chest (if renal function is normal) or a ventilation perfusions (V/Q) scan (if renal function is impaired). The degree of renal impairment that precludes use of IV contrast and thus CT angiography varies by provider, clinical situation, and institutional policy.
These risk stratification tools have not been verified in pregnancy and caution should be given to applying them to these patients. Compared with nonpregnant women, the risk of venous thromboembolism increases fivefold during pregnancy and is
increased by 60-fold in the first 3 months after delivery. VTE in pregnancy is a topic not covered in detail in this chapter.
Diagnostic Testing
Testing is often aimed at determining if there are alternate causes for a patient’s presenting complaint. If there is a high index of suspicion, some of these tests may be omitted. Use of clinical decisions rules can also assist in directing
the workup.
Radiographs
A CXR is useful to rule out other diagnosis such as pneumothorax, congestive heart failure, pneumonia. Infrequently, CXR findings suggest the diagnosis of pulmonary embolism. Hampton’s hump (pulmonary infarct leading to pleural based wedge shaped area of infiltrate), Westermark’s sign (unilateral lung oligemia) and/or unilateral atelectasis may trigger further workup.
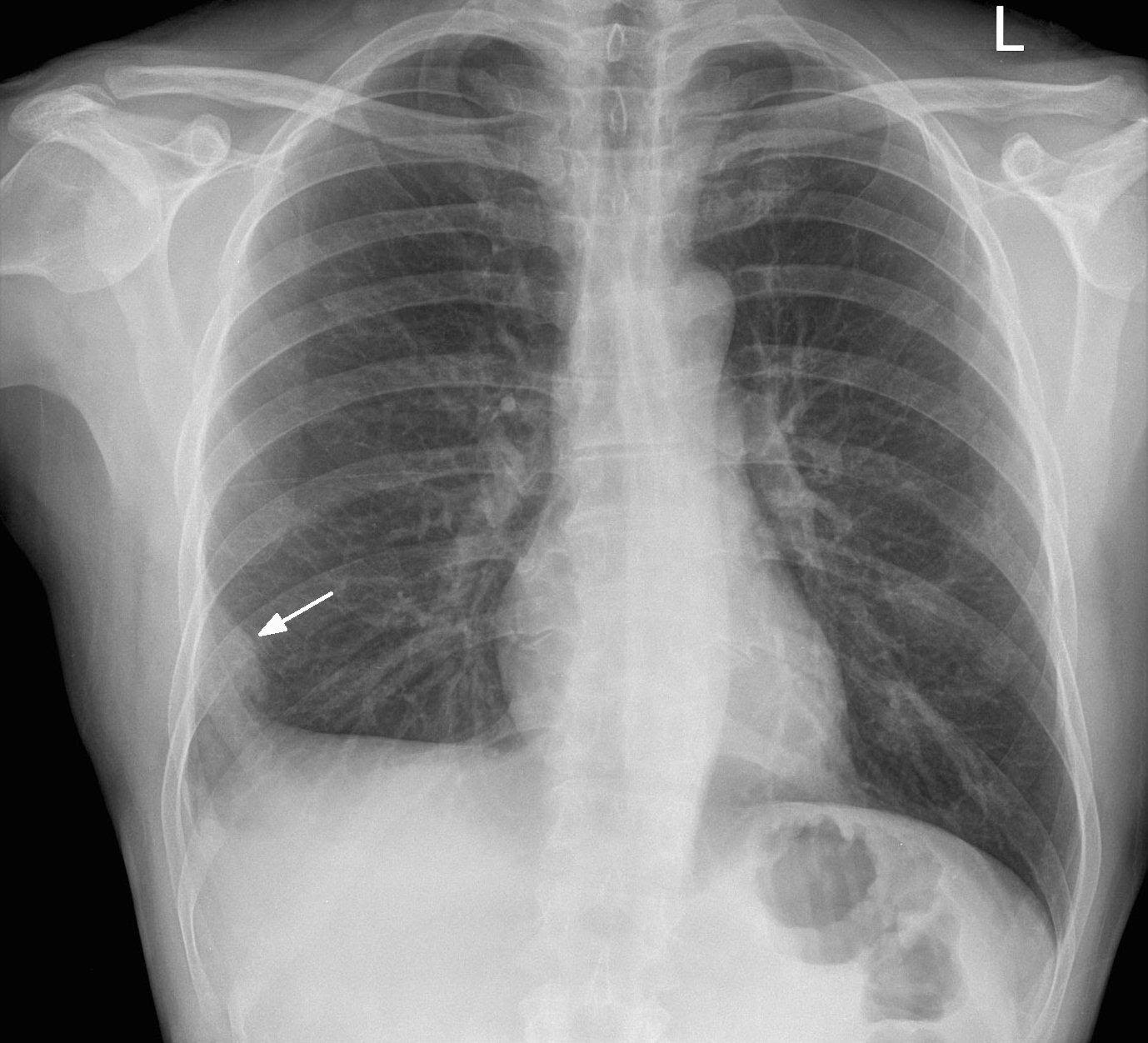 Image 1. PA chest xray in a patient with a PE. Arrow denotes the area of pulmonary infarction, known as Hampton hump. Image courtesy of http://www.imagingpathways.health.wa.gov.au/images/pe/ham.jpg
Image 1. PA chest xray in a patient with a PE. Arrow denotes the area of pulmonary infarction, known as Hampton hump. Image courtesy of http://www.imagingpathways.health.wa.gov.au/images/pe/ham.jpg
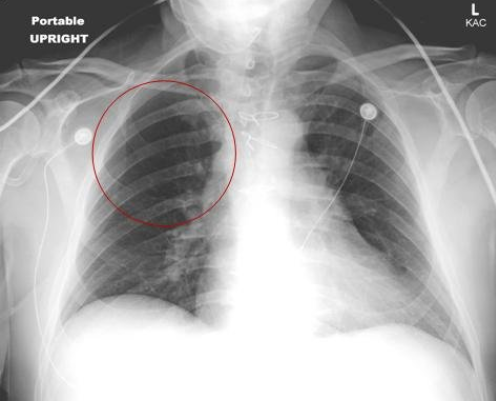
Image 2. Upright portable AP chest x-ray showing hypovolemia secondary to a pulmonary embolism, knowns as Westermark sign. Image courtesy of https://emergencymedicinecases.com/wp-content/uploads/2012/03/015-15-Figure-1.jpg
EKG
EKG findings are usually nonspecific. The most common EKG abnormality is sinus tachycardia, although other findings such as right bundle branch block or evidence of right heart strain (an S wave in lead I and Q and inverted T in lead III, the S1Q3T3 pattern) may be seen.
For a more in-depth discussion and several examples of EKG in patients with PE please see the Life in the Fastlane blog here.
D-dimer
The utility of a d-dimer directly relates to the pretest probability that the patient has a DVT or PE, as determined above by Wells criteria.
D-dimer is a protein derived enzymatic breakdown of cross-linked fibrin. Increased levels indicate the presence of clot formation somewhere in the body. It can be elevated in many diseases, including malignancy, infection, inflammation, MI, stroke, advanced age, and pregnancy and is therefore a very nonspecific test that cannot be used to definitely diagnose any disease process, including DVT and PE. To further complicate matters, there are also several different laboratory techniques of measuring D-dimer that may affect the sensitivity of the test.
Overall, D-dimer generally has good sensitivity and negative predictive value, but poor specificity and positive predictive value. In a setting of a patient with a low to moderate pretest probability, a negative d-dimer virtually rules out
venous thromboembolism. The d-dimer should not be used in patients with a high pre-test probability, as a negative d-dimer in those patients does not result in a posttest probability low enough to comfortably rule out VTE.
Duplex Ultrasonography
Venous duplex ultrasonography is currently the diagnostic test of choice in most centers for DVT. In the hand of an experienced sonographer, the sensitivity and specificity is approximately 95%.
The classic finding on ultrasound for a positive study is the inability to fully compress the vein in the deep venous system of the leg. Other processes (such as a Baker cyst) can also be seen on ultrasound.
For more information and an example of how to perform bedside DVT US click here. There are also several examples of positive DVT US.
CT scan
CT Pulmonary Angiogram (CTPA) is now the accepted study to diagnosis PE in most emergency departments. A CT can also show other possible etiologies, including pneumonia, masses, effusions, aortic dissection or pneumothorax. Risks include the use of iodinated contrast and its potential for contrast-induced nephropathy as well as radiation exposure. Sensitivity of CT scanning is very high but there are limitations. Patients with negative studies who present with high risk of PE or for those whose initial scans are inadequate, should have repeat venous ultrasonography performed to rule out DVT.
CT venography can also be used in conjunction with CT pulmonary angiography to evaluate both for PE and DVT. This has the added risk of increased amounts of IV contrast, and additional radiation to the pelvis and extremities.
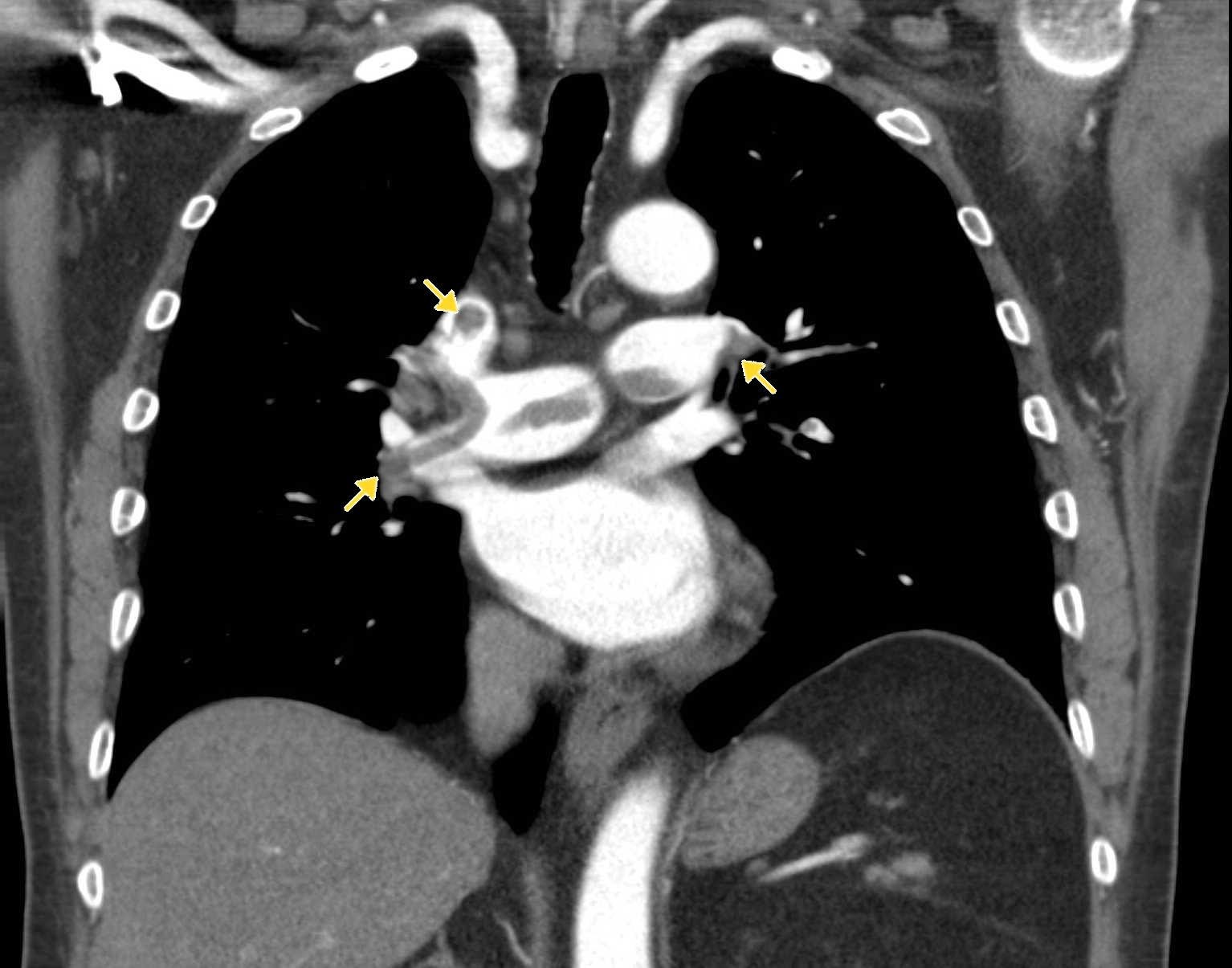
Image 3. CTA pulmonary angiogram. Note the filling defects within the pulmonary vasculature. Image courtesy of http://www.imagingpathways.health.wa.gov.au/images/pe/PE2.jpg
Ventilation Perfusion scan
Patients with a moderate or high pre-test probability for PE should have a imaging study with either CT Pulmonary Angiography (CTPA) or V/Q scan.
To perform a V/Q scan, pictures of the lung are taken while radionuclides are supplied through ventilation (airspace) and perfusion (blood flow). Most areas are both ventilated and perfused. Areas of the lung which are ventilated yet not perfused
represent a mismatch and likely represent pulmonary embolus blocking blood flow. However, various factors affect interpretation of the results, such as preexisting airspace disease, which creates ventilation defects (and therefore possible
perfusion defects), etc.
So how do you make the diagnosis?
All diagnostic labs and imaging should be used in conjunction with your pre-test probability. Although many times a CTPA or V/Q scan will confirm the diagnosis of a pulmonary embolism, false negatives do occur, and interpretation may be subject to the experience of the interpreting radiologist. Therefore a negative CT or V/Q study in a patient with a high pre-test probability should result in further testing or potentially empiric treatment.
Below is a flowchart from Jeff Kline’s paper on diagnostic approach to PE which elucidates the approach outlined previously. This chart references Wells criteria for PE and PERC criteria, which we have discussed here, as well as simplified Revised Geneva Score (sRGS), which we will not cover.
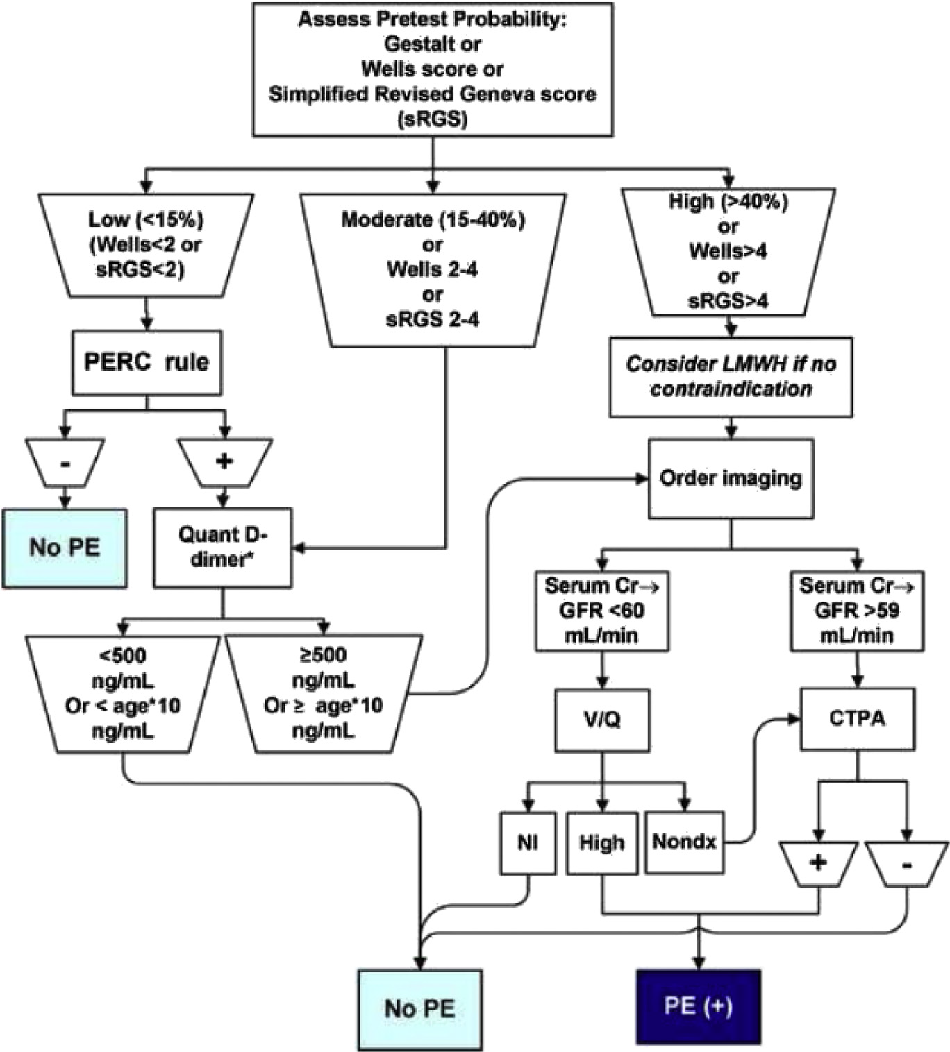
Figure 1. A diagnostic algorithm to in evaluating patients with suspected PE. Original diagram by Dr. Jeff KlineAdapted from @klinelab (September 2014) twitter post. Creative Common 4.0
Treatment
Most patients with confirmed PE or DVT on imaging should be treated with anticoagulation. Anticoagulation may be started before imaging confirmation in patients with a high pre-test probability of the disease. Either unfractionated heparin or low-molecular weight heparins (e.g. enoxaparin) may be used in most cases. Warfarin (Coumadin) has a transient hypercoagulable effect; therefore, patients starting warfarin are placed on heparin until warfarin reaches a therapeutic level (INR 2-3). Classically, patients with confirmed DVT/PE were started on warfarin therapy.
However, with the introduction of DOACs (Direct Oral Anti-coagulants) including rivaroxaban (Xarelto), apixaban (Eliquis) and dabigatran (Pradaxa), alternate strategies to warfarin anticoagulation are now widely prescribed. Most commonly used are rivaroxaban and apixaban. These therapies are ideal for outpatient treatment of DVT/PE because they require no additional monitoring and have a quick onset of action, providing anti-coagulation much sooner than warfarin therapy. The drawback is that these agents are expensive and may not be covered by all insurers.
Contraindications to anticoagulation include patients with active bleeding (cerebral or GI). These patients may benefit from having a inferior vena cava (IVC) filter placed. Contraindications to heparin products include previous reaction to heparins, including heparin-induced thrombocytopenia (HIT). Those with history of reactions to heparin may be started on an oral factor Xa inhibitor, like rivaroxaban or apixaban, or a direct thrombin inhibitor, like argatroban.
Anticoagulation may be withheld in a small subset of patients: those with subsegmental PE in the absence of DVT (confirmed by ultrasound), no evidence of cardiac strain (confirmed by EKG, troponin, and BNP), and no risk factors for thrombosis.
Thrombolytic therapy with alteplase/TPA in the setting of PE is controversial and may be considered in patients with cardiac arrest, hypotension, respiratory failure (hypoxia <90% O2 despite supplemental oxygen, increased work
of breathing), right heart strain on echocardiography, and elevated biomarkers (troponin and BNP) without contraindications to fibrinolysis.
Disposition
In the past, most patients with PE were admitted to the hospital for anticoagulation. Now, patients are risk stratified to determine eligibility for outpatient anticoagulation vs need for inpatient treatment. Two commonly used clinical decision tools are the Simplified Pulmonary Embolism Severity Index (SPESI), and the Hestia Criteria. Low-molecular weight heparin, rivaroxaban and apixaban are all options for outpatient anticoagulation therapy. Patients who are being considered for outpatient therapy should be able to reliably fill their prescriptions and have reliable access to outpatient follow-up.
ICU admission is warranted for patients with “massive” PE and should be considered in patients with “submassive” PE. Massive PE is defined as causing persistent hypotension (including relative hypotension in patients with a history of hypertension) and does not refer to the size of the thrombus itself. Submassive PE is defined as normotensive but with increased work of breathing, hypoxia <90%, new altered mental status, and evidence of right heart strain: elevated troponin or BNP, new RBBB, or evidence of RVs train on echocardiography.
Patients with an isolated DVT without PE sometimes can be set up to receive anticoagulation at home (subcutaneous enoxaparin) with oral anticoagulation (warfarin). This requires teaching and proper coordination with social services
and primary care physicians.
Pearls and Pitfalls
- PE & DVT should be considered as they are common diagnoses with potential fatal outcomes
- Clinical decision rules such as PERC and Wells Criteria help determine pretest probability and guide further workup.
- The D-dimer is a useful diagnostic test in patients with a low to moderate pre-test probability of the disease
- Be familiar with your lab’s d-dimer parameters and cutoffs. Many resources refer to the 500 ng/mL cutoff, but there assays that report results in units of mcg/mL or mg/L.
- Neither the CT nor V/Q scan are 100% sensitive or specific, so patients with a high clinical pre-test probability may be treated or admitted for further diagnostic workup despite initial negative imaging.
Case Study
The patient is risk stratified to a moderate risk category and blood testing returns with a D-Dimer of 650 ng/mL (normal of <500 ng/mL). CTA of the chest demonstrates a right main pulmonary artery occlusion consistent with a pulmonary
embolism. Troponin and BNP biomarkers results negative. Pt has had improvement in his symptoms, has no supplemental oxygen requirement, has proper PCP follow-up and is reliable to take his medications. After discussion with the
patient, and ensuring that he has no contraindications to oral anti-coagulation, the patient is started on apixaban and discharged home.
References
Logan JK, Pantle H, Huiras P, Bessman E, Bright L: Evidence-based diagnosis and thrombolytic treatment of cardiac arrest or periarrest due to suspected pulmonary embolism. Am J Emerg Med 32: 789, 2014. [PubMed: 24856738]
Fengler, BT and Brady, WJ. Fibrinolytic therapy in pulmonary embolism: an evidence-based treatment algorithm. Am J Emerg Med 2009; 27: 84-95.
Kline JA, Courtney DM, Kabrhel C et al. Prospective, multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost 2008; 6: 772-80.
Kline JA, Kabrhel C. Emergency Evaluation for Pulmonary Embolism, Part 2: Diagnostic Approach. The Journal of Emergency Medicine. 2015;49(1):104-117. doi:10.1016/j.jemermed.2014.12.041.
Righini M, Es JV, Exter PD. Age-Adjusted D-Dimer Cutoff Levels to Rule Out Pulmonary Embolism: The ADJUST-PE Study. Journal of Vascular Surgery. 2014;59(5):1469. doi:10.1016/j.jvs.2014.03.260.
Stein PD, Fowler SE, Goodman, LR et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006; 354: 2317.
Van Belle, A, Buller, HR, Huisman, MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172
Wells, PS, Anderson, DR, Rodger, M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med 2001; 135:98.
Kline JA. Venous Thromboembolism. In: Tintinalli JE, Stapczynski J, Ma O, Yealy DM, Meckler GD, Cline DM. eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 8e New York, NY: McGraw-Hill; 2016. http://accessmedicine.mhmedical.com.libproxy.uams.edu/content.aspx?bookid=1658§ionid=109429015. Accessed August 05, 2019.
Young J. Maternal Emergencies After 20 Weeks of Pregnancy and in the Postpartum Period. In: Tintinalli JE, Stapczynski J, Ma O, Yealy DM, Meckler GD, Cline DM. eds.Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 8e New York, NY: McGraw-Hill; 2016. http://accessmedicine.mhmedical.com.libproxy.uams.edu/content.aspx?bookid=1658§ionid=109431050. Accessed August 05, 2019.
